Alzheimer disease infusion clinic Lecanemab Information 
Overview
To be considered for anti-amyloid antibody therapy patients must be referred by specialist neurologists, geriatricians or psychiatrists. It is a triage clinic for patients who are to be considered to be potentially eligible for the new Alzheimer disease anti-amyloid antibody therapies. Patients will also need a GP referral but GP assessment will not be sufficient to be seen.
It is estimated that only about 10-15% of patients who are considered will actually be eligible for infusions through our service, since there are significant risks of this treatment. Please review the eligibility criteria below carefully before considering requesting an appointment.
Lecanemab [Lequembi] is now available under a Special Access Scheme and perhaps via a similar infusion service at Epworth Freemasons form early 2026. MCS does not yet offer Lecanemab infusions at present.
Lecanemab
Lecanemab is for people with early stages of Alzheimer disease before symptoms require much daily support, i.e. patients with mild cognitive impairment or mild dementia. Once Alzheimer disease progresses, lecanemab may no longer be effective so consideration of treatment as soon as possible is important. The Australian TGA has set guidelines on who will be eligible to receive this treatment. Only individuals who meet these criteria will be considered to receive it in our service. These guidelines are based on the trials undertaken with this medication.
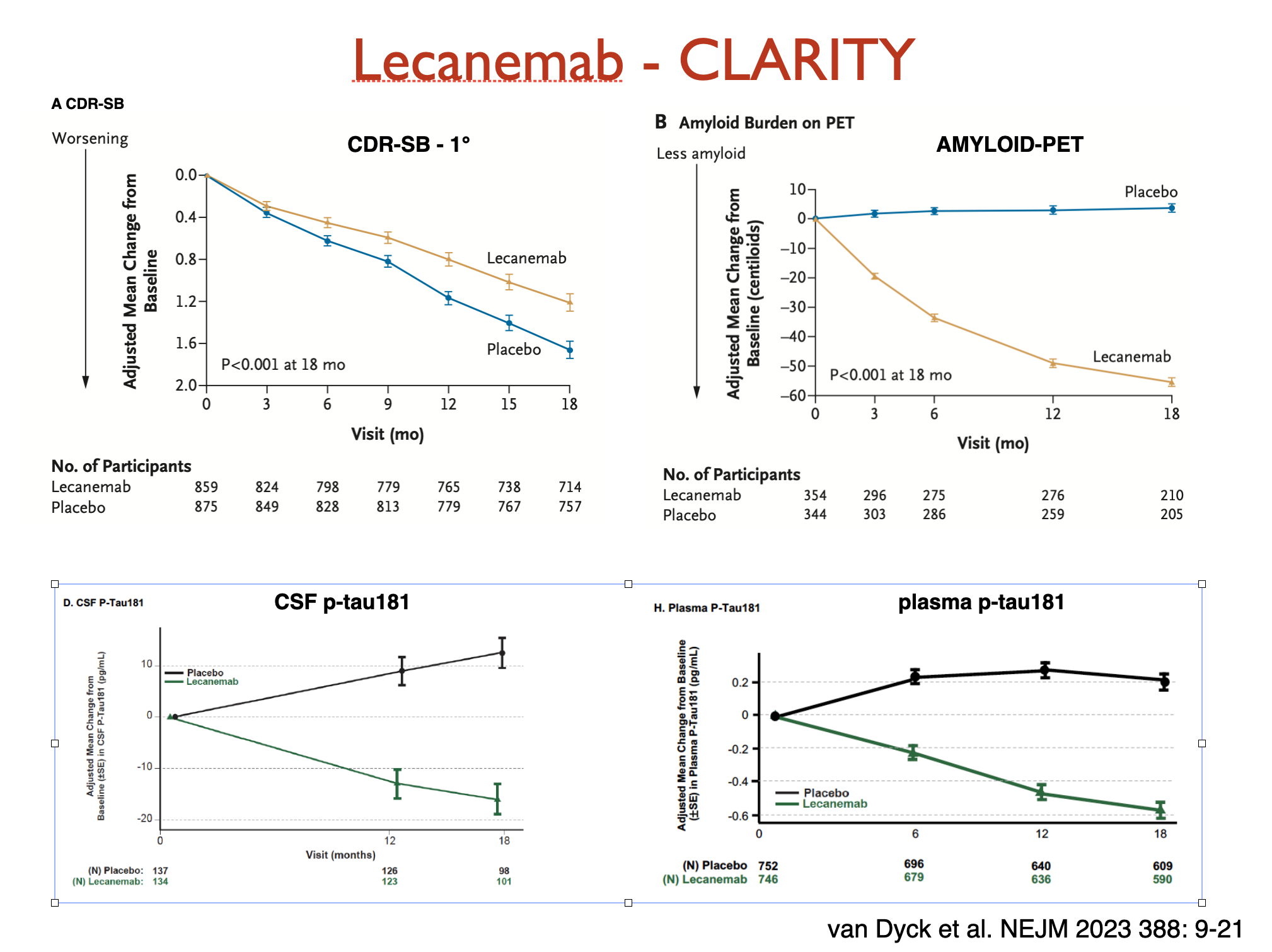
Lecanemab CLARITY AD trial
Lecanemab underwent several trials including the phase 3 CLARITY AD was published in the New England Journal of Medicine on 5 Jan 2023. It was a double-blind placebo-controlled randomized trial of 1795 participants with ~50% on 10mg/kg lecanemab and 50% on placebo every 2 weeks for 18 months.
It is given by infusion (into a vein via an intravenous drip) over about 2 hours.
Participants were eligible if:
- They had mild cognitive impairment or mild dementia due to Alzheimer disease
- They were age 50-90 inclusive
- There was minimal functional impairment
- Mini-mental state examination (MMSE) score was > 22/30
Participants were NOT eligible if:
- There was MRI evidence of significant brain haemorrhages (including microbleeds), strokes, severe white matter disease (related to small vessel ischaemia)
- There were significant other neurological, psychiatric or medical conditions
Results:
- All participants continued to decline, ie treatment did not stop decline
- Lecanemab treated patients declined slightly slower (27% reduction, with modelling showing an "average" of 5 months less decline over the 18 months)
- Amyloid on PET scanning, and both CSF and plasma ptau-181 showed improvements
- Amyloid decreased markedly on PET scanning (amyloid is the target of the antibody)
- A marker of neurodegeneration (ptau-181) also reduced markedly in the lecanemab group
- Subgroups: Some groups did not show statistical benefit. These had small numbers of participants so it is not certain if they would individually respond to treatment. These included Asian, female and younger (< 65 years of age) participants.
Adverse effects:
- ARIA stands for "amyloid related imaging abnormalities" which are either oedema (ARIA-E, reversible brain swelling) or haemorrhages (ARIA-H, typically small microbleeds in the cortex or outer layers of the brain, but also on the surface ['siderosis'] or within the lobes ("lobar")).
- ARIA is detectable using MRI (including special sequences sensitive to haemorrhage).
- MRIs are recommended for detecting ARIA 1-3 monthly during the first year.
- ARIA-H occurred in the placebo treated group as well so is a part of Alzheimer disease in some people even without anti-amyloid antibody therapy.
- ARIA-H occurs in treated people also within areas of ARIA-E.
- ARIA usually occurs without any symptoms and does not cause persisting impairment. The ARIA-E resolves, but the result of any haemorrhage (residual tissue iron deposits) remain permanently and are detected on MRI also.
- ARIA usually occurs in the first 3-6 months, and typically resolves within 4 months.
- Management depends on severity, and presence of symptoms. Most ARIA is without symptoms. Management can include continuing, postponing or stopping infusions, and sometimes medication (steroids) to reduce swelling.
- ARIA occurred in 14% of patients overall (ARIA-E 13%) only 3.3% had symptoms
- ARIA occurred more in those with apolipoprotein E4/E4 status - 39%
- Mild symptoms: headache, confusion, nausea, dizziness
- Serious symptoms: focal "stroke-like" deficits, seizures, more marked brain impairment or death
- There were 3 deaths in this trial thought due to the drug causing ARIA, and one further death in a participant who suffered a stroke, and was given clot-busting therapy (tPA) leading to massive intracerebral haemorrhage.
- There have been 3 deaths in the Open Label Extension of the CLARITY study as well (out of 900 participants) emphasizing that this is not a benign treatment (and patients are generally older so more prone to co-morbid conditions that might lead also to death).
- Infusion reactions occurred in 26% of patient (self-limited).
Please go back to the prior page for eligibility criteria to consider this or other therapies to be given by MCS.